Textbook Question
Convert the following models into a condensed structures and line drawings.
(a)

 Verified step by step guidance
Verified step by step guidance
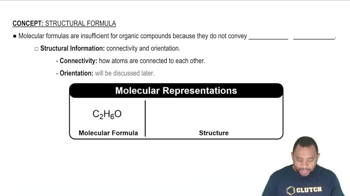
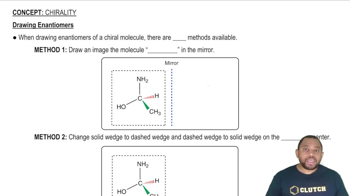
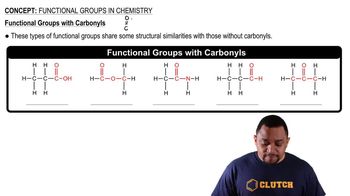
Convert the following models into a condensed structures and line drawings.
(a)
Convert the following model into a condensed structure and line drawing. Draw the structures of two isomeric compounds.
Propose structures and draw condensed formulas for molecules that meet the following descriptions.
(a) A ketone with the formula C5H10
Propose structures and draw condensed formulas for molecules that meet the following descriptions.
(b) An ester with the formula C6H12O2
Propose structures and draw condensed formulas for molecules that meet the following descriptions.
(c) A compound with formula C2H5NO2 that is both an amine and a carboxylic acid