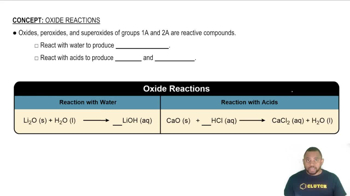
Oxides and Their Reactions with Water
Oxides are compounds formed by the reaction of an element with oxygen. When certain oxides react with water, they can form either acids or bases, depending on their nature. For example, metal oxides typically produce basic solutions, while non-metal oxides often yield acidic solutions, which is essential for predicting the products of the reactions in the question.

 Verified step by step guidance
Verified step by step guidance