Ch.22 - The Main Group Elements

Chapter 22, Problem 38
Which element in each of the following pairs has more metallic character? (a) Bi or As
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of metallic character. Metallic character refers to how easily an element can lose electrons to form positive ions (cations). Elements with higher metallic character are typically more reactive metals.
Step 2: Locate the elements on the periodic table. Bismuth (Bi) and Arsenic (As) are both in Group 15 of the periodic table, with Bi being below As.
Step 3: Recall the trend of metallic character in the periodic table. Metallic character increases as you move down a group and decreases as you move across a period from left to right.
Step 4: Apply the trend to the given elements. Since Bi is below As in the same group, Bi has more metallic character than As.
Step 5: Conclude that Bismuth (Bi) has more metallic character than Arsenic (As) based on its position in the periodic table.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Metallic Character
Metallic character refers to the tendency of an element to exhibit properties typical of metals, such as conductivity, malleability, ductility, and luster. It increases down a group in the periodic table and decreases across a period from left to right. Understanding this concept is crucial for comparing elements, as it helps predict their behavior and reactivity.
Recommended video:
Guided course
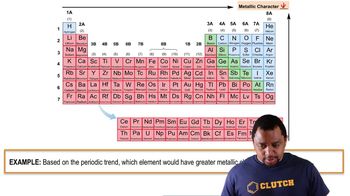
Metallic Character Example
Periodic Trends
Periodic trends are patterns observed in the properties of elements as you move across or down the periodic table. Key trends include atomic radius, ionization energy, electronegativity, and metallic character. Recognizing these trends allows for informed predictions about the properties of elements, such as which element in a pair is more metallic.
Recommended video:
Guided course
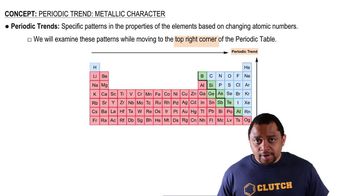
Periodic Trends
Position in the Periodic Table
The position of an element in the periodic table significantly influences its properties, including metallic character. Elements located towards the left and bottom of the table tend to be more metallic, while those on the right and top are more non-metallic. In the case of bismuth (Bi) and arsenic (As), their positions help determine which has greater metallic character.
Recommended video:
Guided course
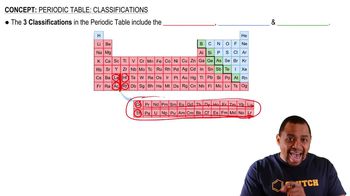
Periodic Table Classifications
Related Practice
