Write a balanced net ionic equation for the reaction of the amphoteric oxide ZnO with:
a. Hydrochloric acid

 Verified step by step guidance
Verified step by step guidance
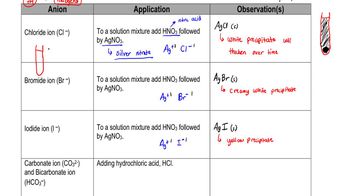

Write a balanced net ionic equation for the reaction of the amphoteric oxide ZnO with:
a. Hydrochloric acid
In the following pictures of binary hydrides, ivory spheres
represent H atoms or ions, and burgundy spheres represent
atoms or ions of the other element.
(1)
(2)
(3)
(4)
(a) Identify each binary hydride as ionic, covalent, or interstitial.
(b) What is the oxidation state of hydrogen in compounds (1), (2), and (3)? What is the oxidation state of the other
element?
Consider the six second- and third-row elements in groups 4A–6A of the periodic table:
Possible structures for the binary fluorides of each of these elements in its highest oxidation state are shown below.
(a) Identify the nonfluorine atom in each case, and write the molecular formula of each fluoride.
The following pictures represent structures of the hydrides of four second-row elements:
(1)
(2)
(3)
(4)
(b) Which compound has the lowest boiling point?
The following models represent the structures of binary hydrides of second-row elements:
b. Draw an electron-dot structure for each hydride. For which hydride is there a problem in drawing the structure? Explain.
Look at the location of elements A, B, C, and D in the following periodic table:
Which hydride has the lowest boiling point?