Which element in each of the following pairs has more nonmetallic character?
(a) Se or Te

 Verified step by step guidance
Verified step by step guidance
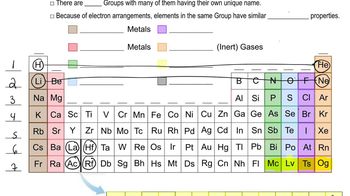

Which element in each of the following pairs has more nonmetallic character?
(a) Se or Te
Write a balanced net ionic equation for the reaction of the amphoteric oxide ZnO with:
a. Hydrochloric acid
In the following pictures of binary hydrides, ivory spheres
represent H atoms or ions, and burgundy spheres represent
atoms or ions of the other element.
(1)
(2)
(3)
(4)
(a) Identify each binary hydride as ionic, covalent, or interstitial.
(b) What is the oxidation state of hydrogen in compounds (1), (2), and (3)? What is the oxidation state of the other
element?
The following pictures represent various silicate anions. Write the formula and charge of each anion.
The following pictures represent structures of the hydrides of four second-row elements:
(1)
(2)
(3)
(4)
(b) Which compound has the lowest boiling point?
The following models represent the structures of binary hydrides of second-row elements:
b. Draw an electron-dot structure for each hydride. For which hydride is there a problem in drawing the structure? Explain.