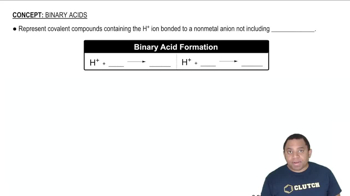
Naming Acids
Naming acids involves understanding the relationship between the anhydride and the resulting acid. For oxoacids, the name is derived from the central atom and the number of oxygen atoms. In this case, the reaction of I2O5 with water produces iodic acid (HIO3), which is named based on the presence of iodine and the oxidation state of the iodine in the compound.

 Verified step by step guidance
Verified step by step guidance