Textbook Question
Which compound in each of the following pairs is more ionic?
(b) P4O6 or Ga2O3

 Verified step by step guidance
Verified step by step guidance

Which compound in each of the following pairs is more ionic?
(b) P4O6 or Ga2O3
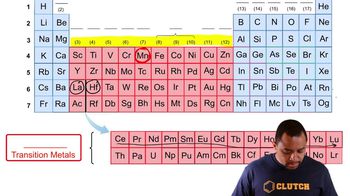
Look at the location of elements A, B, C, and D in the following periodic table:
(b) Classify each oxide as basic, acidic, or amphoteric.
Identify the group 3A element that best fits each of the following descriptions.
(c) Is extremely toxic
Consider the elements N, Si, Al, S, and F. Identify which of these elements:
c. Is a semiconductor
Which compound in each of the following pairs is more ionic?
(d) BCl3 or AlCl3
Give one example from main-group chemistry that illustrates each of the following descriptions.
d. Polar molecule that violates the octet rule