Textbook Question
Complete and balance the equation for each of the following reactions.
b. Ca(s) + H2O(l) →

 Verified step by step guidance
Verified step by step guidance
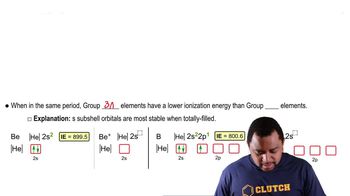


Complete and balance the equation for each of the following reactions.
b. Ca(s) + H2O(l) →
Which compound in each of the following pairs is more ionic?
(b) P4O6 or Ga2O3
Look at the location of elements A, B, C, and D in the following periodic table:
(b) Classify each oxide as basic, acidic, or amphoteric.
Consider the elements C, Se, B, Sn, and Cl. Identify which of these elements:
c. Is the best electrical conductor
Consider the elements N, Si, Al, S, and F. Identify which of these elements:
c. Is a semiconductor
Which compound in each of the following pairs is more ionic?
(d) BCl3 or AlCl3