Ch.22 - The Main Group Elements

Chapter 22, Problem 164
Consider phosphorous acid, a polyprotic acid with the formula H3PO3. (a) Draw two plausible structures for H3PO3. For each one, predict the shape of the pH titration curve for the titration of H3PO3 (Ka1 = 1.0 * 10^-2) with aqueous NaOH. (b) For the structure with the H atoms in two different environments, calculate the pH at the first and second equivalence points, assuming that 30.00 mL of 0.1240 M H3PO3 (Ka2 = 2.6 * 10^-7) is titrated with 0.1000 M NaOH.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Draw two plausible structures for H3PO3. Consider the possible arrangements of hydrogen atoms and the central phosphorus atom, keeping in mind that phosphorous acid is a polyprotic acid, meaning it can donate more than one proton.
Step 2: Analyze the structures to determine the environments of the hydrogen atoms. Identify which hydrogen atoms are acidic and can be donated during the titration process.
Step 3: Predict the shape of the pH titration curve for each structure. Consider the number of acidic protons and their respective dissociation constants (Ka1 and Ka2) to understand how the pH changes as NaOH is added.
Step 4: Calculate the pH at the first equivalence point. Use the initial concentration of H3PO3 and the concentration of NaOH to determine the volume of NaOH needed to reach the first equivalence point, then apply the appropriate equilibrium expression to find the pH.
Step 5: Calculate the pH at the second equivalence point. Consider the remaining acidic hydrogen and its dissociation constant (Ka2) to determine the pH after the second equivalence point is reached.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Polyprotic Acids
Polyprotic acids are acids that can donate more than one proton (H+) per molecule in a stepwise manner. Each dissociation step has its own acid dissociation constant (Ka), which indicates the strength of the acid at that stage. For phosphorous acid (H3PO3), the first dissociation is stronger than the second, leading to different pH behaviors during titration. Understanding the dissociation process is crucial for predicting the pH at various points in a titration curve.
Recommended video:
Guided course

Polyprotic Buffers
Titration Curves
A titration curve is a graphical representation of the pH of a solution as a function of the volume of titrant added. For polyprotic acids, the curve typically shows multiple inflection points corresponding to the different dissociation steps. The shape of the curve is influenced by the strength of the acid and the concentration of the titrant. Analyzing the curve helps in identifying equivalence points, where the amount of titrant equals the amount of acid present.
Recommended video:
Guided course
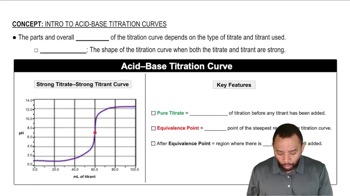
Acid-Base Titration Curves
Equivalence Points
Equivalence points in a titration occur when the amount of titrant added is stoichiometrically equivalent to the amount of acid present in the solution. For polyprotic acids like H3PO3, there are multiple equivalence points corresponding to each proton donation. The pH at these points can be calculated using the concentrations of the acid and the base, as well as the dissociation constants. Understanding how to calculate pH at these points is essential for accurately interpreting titration results.
Recommended video:
Guided course
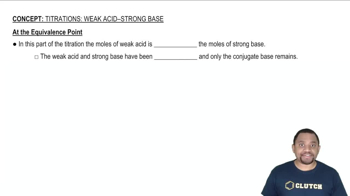
At the Equivalence Point
Related Practice
