Textbook Question
Write chemical equations for the reaction of calcium with the following substances, making sure that the numbers and kinds of atoms are the same on both sides of the equations. If no reaction occurs, write N.R.
(c) Br2

 Verified step by step guidance
Verified step by step guidance

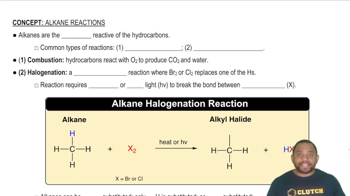
Write chemical equations for the reaction of calcium with the following substances, making sure that the numbers and kinds of atoms are the same on both sides of the equations. If no reaction occurs, write N.R.
(c) Br2
Write chemical equations for the reaction of calcium with the following substances, making sure that the numbers and kinds of atoms are the same on both sides of the equations. If no reaction occurs, write N.R.
(d) O2
Milk of magnesia, a widely used antacid, is an aqueous suspension of Mg1OH22. How would you prepare Mg1OH22 from magnesium metal?