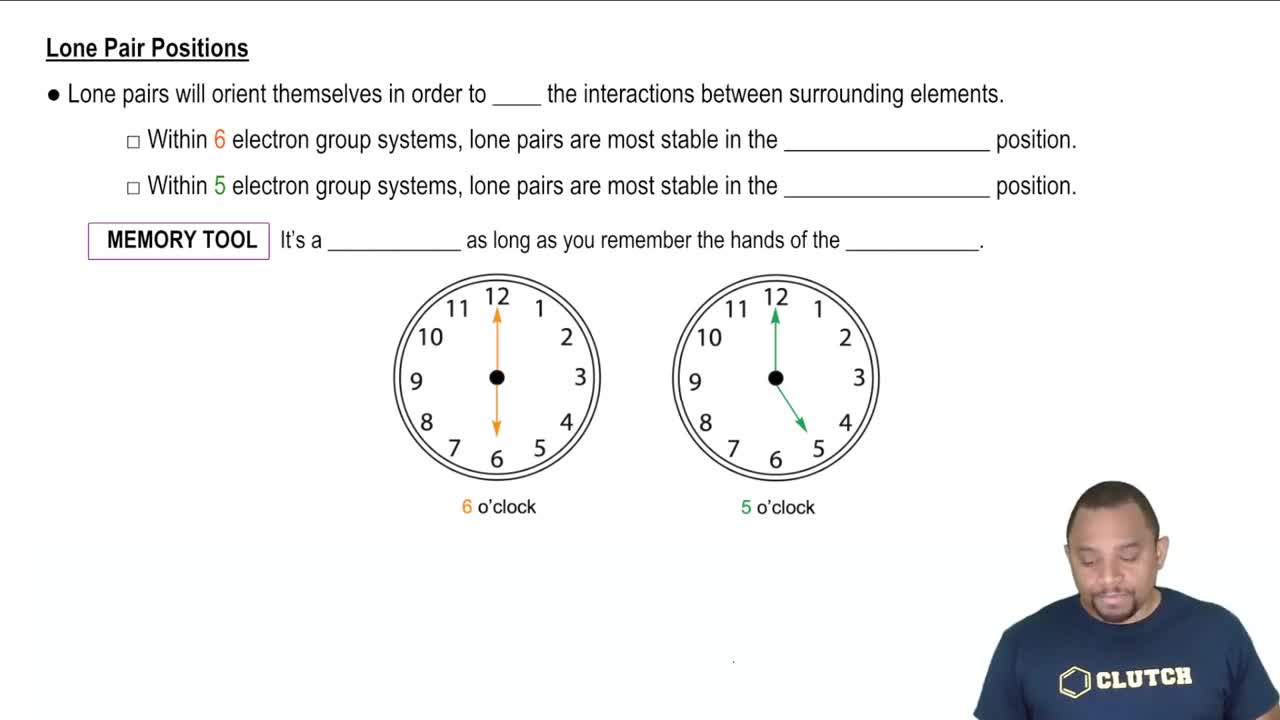
Textbook Question
Look at the location of elements A, B, C, and D in the following periodic table:
(a) Write the formula of the oxide that has each of these elements in its highest oxidation state.

 Verified step by step guidance
Verified step by step guidance

Look at the location of elements A, B, C, and D in the following periodic table:
(a) Write the formula of the oxide that has each of these elements in its highest oxidation state.
The following models represent the structures of binary
oxides of second- and third-row elements in their highest
oxidation states:
(a) Identify the non-oxygen atom in each case, and write the molecular formula for each oxide.