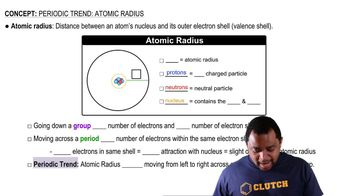
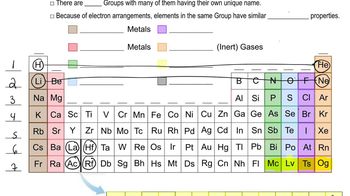
Textbook Question
The following models represent the structures of binary
oxides of second- and third-row elements in their highest
oxidation states:
(a) Identify the non-oxygen atom in each case, and write the molecular formula for each oxide.

 Verified step by step guidance
Verified step by step guidance