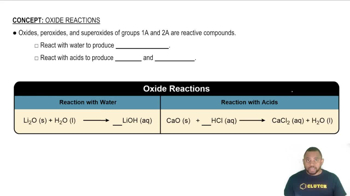
Trends in Acid-Base Character
The acid-base character of oxides generally increases with the nonmetallic character of the element. As you move from left to right across the periodic table, oxides become more acidic, while moving down a group typically leads to more basic oxides. This trend helps in predicting the acidic character of the given oxides based on their elemental composition.

 Verified step by step guidance
Verified step by step guidance