Tris(2-aminoethyl)amine, abbreviated tren, is the tetradentate ligand N(CH2CH2NH2)3. Using to represent each of the three NCH2CH2NH2 segments of the ligand, sketch all possible isomers of the octahedral complex [Co(tren)BrCl]+.

Use a sketch to explain why the dxy and dx2-y2 orbitals have different energies in an octahedral complex. Which of the two orbitals has higher energy?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Crystal Field Theory

d-Orbital Splitting in Octahedral Fields
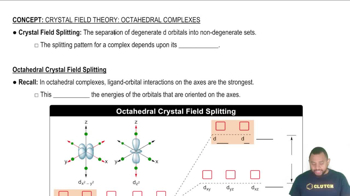
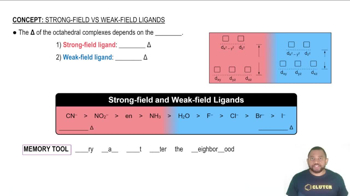
Ligand Field Strength
Consider the octahedral complex [Co(en)(dien)Cl]2+, where dien = H2NCH2CH2NHCH2CH2NH2, which can be abbreivated
(a) The dien (diethylenetriamine) ligand is a tridentate ligand. Explain what is meant by 'tridentate' and why dien can act as a tridentate ligand.
(b) Draw all possible stereoisomers of [Co(en)(dien)Cl]2+ (dien is a flexible ligand). Which stereoisomers are chiral, and which are achiral?
The reaction of the octahedral complex Co(NH3)3(NO2)3 with HCl yields a complex [Co(NH3)3(H2O)Cl2]+ in which the two chloride ligands are trans to one another.
(a) Draw the two possible stereoisomers of the starting material [Co(NH3)3(NO2)3]. (All three NO2- ligands are bonded to Co through the N atom.)
(b) Assuming that the NH3 groups remain in place, which of the two starting isomers could give rise to the observed product?
In octahedral complexes, the choice between high-spin and low-spin electron configurations arises only for d4 - d7 complexes. Explain.
