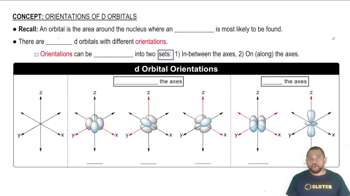
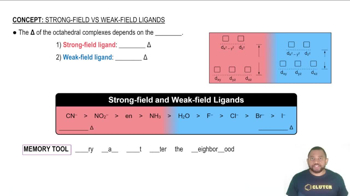
Consider the octahedral complex [Co(en)(dien)Cl]2+, where dien = H2NCH2CH2NHCH2CH2NH2, which can be abbreivated
(a) The dien (diethylenetriamine) ligand is a tridentate ligand. Explain what is meant by 'tridentate' and why dien can act as a tridentate ligand.
(b) Draw all possible stereoisomers of [Co(en)(dien)Cl]2+ (dien is a flexible ligand). Which stereoisomers are chiral, and which are achiral?