Draw the structure of the iron oxalate complex [Fe(C2O4)3]3-. Describe the coordination geometry, and identify any chelate rings. What are the coordination number and the oxidation number of the iron?
Ch.21 - Transition Elements and Coordination Chemistry

Chapter 21, Problem 72b
Draw the structure of the following complexes. What are the oxidation state, coordination number, and coordination geometry of the metal in each?
(b) [Cr(NH3)2(C2O4)2]NO2
 Verified step by step guidance
Verified step by step guidance1
Determine the oxidation state of Cr: Assume the oxidation state of Cr is x. Ammonia (NH3) is a neutral ligand, and oxalate (C2O4) is a bidentate ligand with a charge of -2. The complex is overall neutral, so x + 2(0) + 2(-2) = 0. Solve for x.
Identify the coordination number: Count the number of ligand donor atoms attached to the metal. Each NH3 contributes 1 donor atom, and each C2O4 contributes 2 donor atoms. Calculate the total.
Determine the coordination geometry: Based on the coordination number, predict the geometry. Common geometries include octahedral, tetrahedral, and square planar.
Draw the structure: Arrange the ligands around the metal center according to the determined geometry. Indicate the bidentate nature of the oxalate ligands.
Consider the counterion: Note that NO2 is the counterion and does not affect the coordination sphere of the complex.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation State
The oxidation state of an element in a compound indicates the degree of oxidation of that element, reflecting the number of electrons lost or gained. In coordination complexes, the oxidation state of the metal can be determined by considering the charges of the ligands and the overall charge of the complex. For example, in [Cr(NH3)2(C2O4)2]NO2, the oxidation state of chromium can be calculated based on the charges contributed by the ammonia and oxalate ligands.
Recommended video:
Guided course

Oxidation Numbers
Coordination Number
The coordination number refers to the total number of ligand atoms that are directly bonded to the central metal atom in a coordination complex. It is determined by the number of donor atoms from the ligands that coordinate to the metal. In the case of [Cr(NH3)2(C2O4)2], the coordination number can be assessed by counting the donor atoms from the two NH3 ligands and the two oxalate ligands.
Recommended video:
Guided course

Coordination Numbers
Coordination Geometry
Coordination geometry describes the spatial arrangement of the ligands around the central metal atom in a coordination complex. Common geometries include octahedral, tetrahedral, and square planar, which depend on the coordination number and the steric and electronic properties of the ligands. For [Cr(NH3)2(C2O4)2], the coordination geometry can be inferred from the coordination number and the nature of the ligands involved.
Recommended video:
Guided course
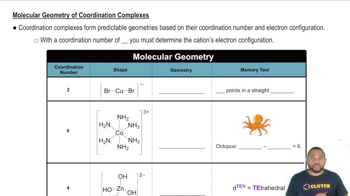
Molecular Geometry of Coordination Complexes
Related Practice
Textbook Question
Textbook Question
Draw the structure of the following complexes. What are the oxidation state, coordination number, and coordination geometry of the metal in each?
(a) Na[Au(CN)2]
Textbook Question
Draw the structure of the following complexes. What are the oxidation state, coordination number, and coordination geometry of the metal in each?
(a) Pt(en)2
Textbook Question
Draw the structure of the following complexes. What are the oxidation state, coordination number, and coordination geometry of the metal in each?
(b) [Co(H2O)5Cl]SO4]
Textbook Question
(a) Identify the Lewis acid and the Lewis base in the reaction of ethylenediamine (H2NCH2CH2NH2) with Ni2+ to form [Ni(en)3]2+.
(b) Identify the ligands and donor atoms.
(c) Give the coordination number and geometry of the metal in the complex.
