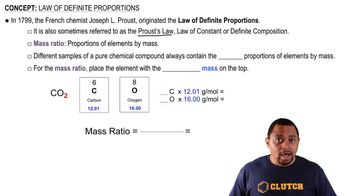
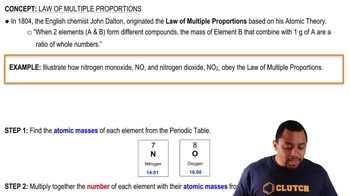
Textbook Question
A sample of mercury with a mass of 114.0 g was combined with 12.8 g of oxygen gas, and the resulting reaction gave 123.1 g of mercury(II) oxide. How much oxygen was left over after the reaction was complete?

 Verified step by step guidance
Verified step by step guidance