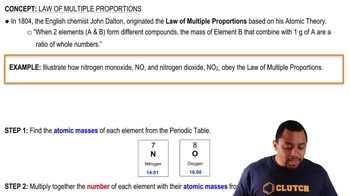
Textbook Question
A sample of CaCO3 was heated, causing it to form CaO and CO2 gas. Solid CaO remained behind, while the CO2 escaped to the atmosphere. If the CaCO3 weighed 612 g and the CaO weighed 343 g, how many grams of CO2 were formed in the reaction?