Textbook Question
Benzene, ethane, and ethylene are just three of a large num-ber of hydrocarbons—compounds that contain only carbon and hydrogen. Show how the following data are consistent with the law of multiple proportions.

 Verified step by step guidance
Verified step by step guidance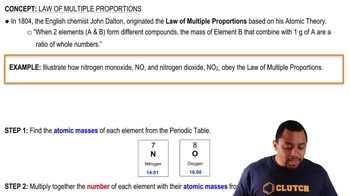

Label the following statements about J. J. Thomson's cathode-ray tube experiments shown in Figure 2.6 as true or false. (b) A cathode ray is a stream of charged particles.