Textbook Question
(b) The mole is a unit used to represent a very large number of atoms. How many atoms are equivalent to 1 mol of atoms?

 Verified step by step guidance
Verified step by step guidance

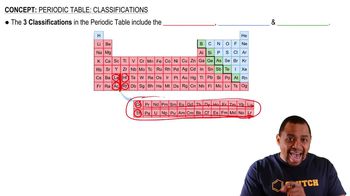
(b) The mole is a unit used to represent a very large number of atoms. How many atoms are equivalent to 1 mol of atoms?
Naturally occurring silver consists of two isotopes: 107^Ag (51.84%) with an isotopic mass of 106.9051 and 109^Ag (48.16%) with an isotopic mass of 108.9048. What is the atomic weight of silver? Check your answer in a periodic table.