Textbook Question
(a) The unified atomic mass unit (u) is used to represent the extremely small mass of atoms. How many grams are equivalent to 1 u?

 Verified step by step guidance
Verified step by step guidance

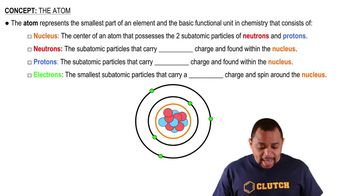

(a) The unified atomic mass unit (u) is used to represent the extremely small mass of atoms. How many grams are equivalent to 1 u?
(b) The mole is a unit used to represent a very large number of atoms. How many atoms are equivalent to 1 mol of atoms?