Textbook Question
Balance the following equations for the nuclear fission of 235U. (a)
1
views

 Verified step by step guidance
Verified step by step guidance
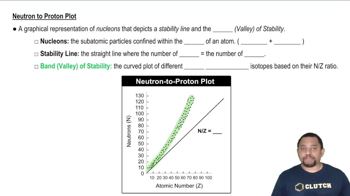

Balance the following equations for the nuclear fission of 235U. (a)
Balance the following equations for the nuclear fission of 235U. (b)