Textbook Question
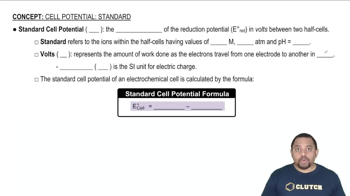
What conditions must be met for a cell potential E to qualify as a standard cell potential E°?
3
views

 Verified step by step guidance
Verified step by step guidance

An H2/H+ half-cell (anode) and an Ag+/Ag half cell (cathode) are connected by a wire and a salt bridge. (a) Sketch the cell, indicating the direction of electron and ion flow.
An H2/H+ half-cell (anode) and an Ag+/Ag half cell (cathode) are connected by a wire and a salt bridge. (b) Write balanced equations for the electrode and overall cell reactions.
An H2/H+ half-cell (anode) and an Ag+/Ag half cell (cathode) are connected by a wire and a salt bridge. (c) Give the shorthand notation for the cell.