Textbook Question
Which state in each of the following pairs has the higher entropy per mole of substance? (d) CO2 at STP or CO2 at 100 °C and 0.1 atm

 McMurry 8th Edition
McMurry 8th Edition Ch.18 - Thermodynamics: Entropy, Free Energy & Equilibrium
Ch.18 - Thermodynamics: Entropy, Free Energy & Equilibrium Problem 57d
Problem 57d Verified step by step guidance
Verified step by step guidance

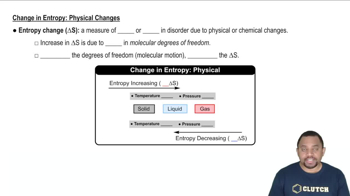

Which state in each of the following pairs has the higher entropy per mole of substance? (d) CO2 at STP or CO2 at 100 °C and 0.1 atm
Which state in each of the following pairs has the higher entropy per mole of substance? (a) Ice at -40 °C or ice at 0 °C
Which state in each of the following pairs has the higher entropy per mole of substance? (b) N2 at STP or N2 at 0 °C and 10 atm