Textbook Question
Phosphorus pentachloride forms from phosphorus trichloride and chlorine:
(a) Use data in Appendix B to calculate ∆Ssys, ∆Ssurr, and ∆Stotal for this reaction. Is the reaction spontaneous under standard-state conditions at 25 °C?

 McMurry 8th Edition
McMurry 8th Edition Ch.18 - Thermodynamics: Entropy, Free Energy & Equilibrium
Ch.18 - Thermodynamics: Entropy, Free Energy & Equilibrium Problem 72
Problem 72 Verified step by step guidance
Verified step by step guidance
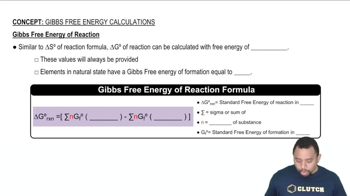
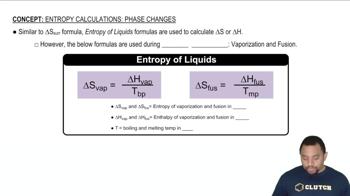
Phosphorus pentachloride forms from phosphorus trichloride and chlorine:
(a) Use data in Appendix B to calculate ∆Ssys, ∆Ssurr, and ∆Stotal for this reaction. Is the reaction spontaneous under standard-state conditions at 25 °C?
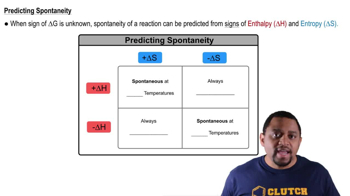
Phosphorus pentachloride forms from phosphorus trichloride and chlorine: PCl3(g) + Cl2(g) → PCl5(g) (b) Estimate the temperature at which the reaction will become nonspontaneous.