Textbook Question
A 100.0 mL sample of a solution that is 0.100 M in HCl and 0.100 M in HCN is titrated with 0.100 M NaOH. Calculate the pH after the addition of the following volumes of NaOH: (b) 75.0 mL

 Verified step by step guidance
Verified step by step guidance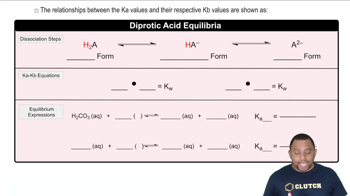
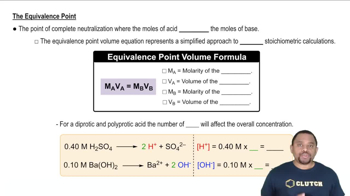

A 40.0 mL sample of a mixture of HCl and H3PO4 was titrated with 0.100 M NaOH. The first equivalence point was reached after 88.0 mL of base, and the second equivalence point was reached after 126.4 mL of base. (a) What is the concentration of H3O+ at the first equivalence point?