pH and Acid-Base Equilibrium
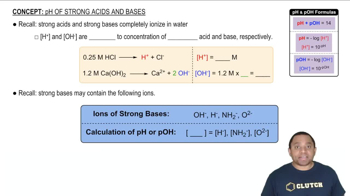
pH is a measure of the acidity or basicity of a solution, with lower values indicating higher acidity. In the context of urine, the pH affects the ionization of weak acids like oxalic acid, which can dissociate into oxalate ions (C2O4^2-) that participate in the formation of calcium oxalate precipitate. The degree of ionization is influenced by the pH, and a lower pH (like 5.5) favors the formation of oxalate ions, potentially leading to precipitation if the ion concentrations exceed the Ksp.

 Verified step by step guidance
Verified step by step guidance