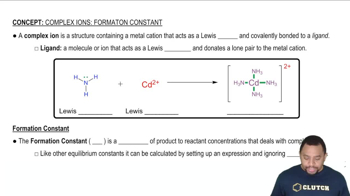
Textbook Question
Is the solubility of Zn(OH)2 increased, decreased, or unchanged on addition of each of the following substances? Write a balanced net ionic equation for each dissolution reaction. (See Appendix C.6 for formulas of complex ions.) (b) KOH