Concentration and Dilution
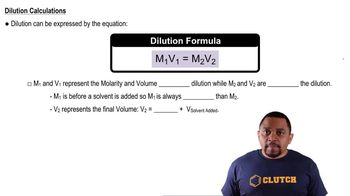
Concentration refers to the amount of a substance in a given volume of solution, typically expressed in molarity (M). When mixing solutions, the final concentration of ions can change due to dilution or the addition of solutes. In this question, calculating the final concentration of carbonate ions after adding Na2CO3 is vital to determine if it will lead to the formation of CaCO3 precipitate.

 Verified step by step guidance
Verified step by step guidance