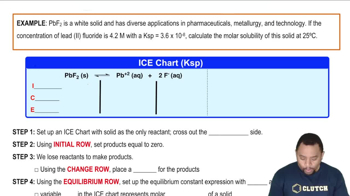
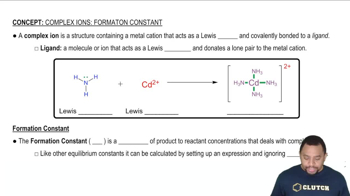
Textbook Question
Write a balanced net ionic equation for each of the follow-ing dissolution reactions, and use the appropriate Ksp and Kf values in Appendix C to calculate the equilibrium constant for each.(a) AgI in aqueous NaCN to form [Ag(CN)2]-

 Verified step by step guidance
Verified step by step guidance