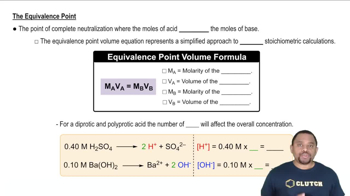
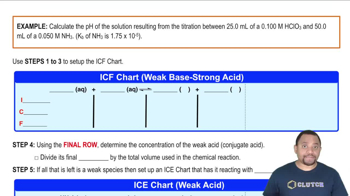
Textbook Question
Consider the titration of 60.0 mL of 0.150 M HNO3 with 0.450 M NaOH.(a) How many millimoles of HNO3 are present at the start of the titration?(b) How many milliliters of NaOH are required to reach the equivalence point?(c) What is the pH at the equivalence point? (d) Sketch the general shape of the pH titration curve.