Textbook Question
Calculate the H3O+ concentration to the correct number of significant figures for solutions with the following pH values. (d) 14.25


 Verified step by step guidance
Verified step by step guidance


Calculate the H3O+ concentration to the correct number of significant figures for solutions with the following pH values. (d) 14.25
Calculate the H3O+ concentration to the correct number of significant figures for solutions with the following pH values. (e) -1.0
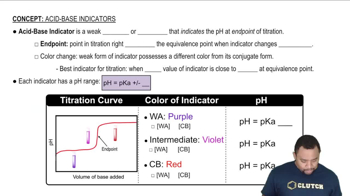
Which of the indicators given in Figure 16.5, methyl violet, bromcresol green, phenol red, or thymolphthalein, would be most appropriate to detect a pH change from: (b) 8 to 10?