Textbook Question
Identify the stronger base in each of the following pairs.
Explain your reasoning.
(c) HS- or OH-

 Verified step by step guidance
Verified step by step guidance
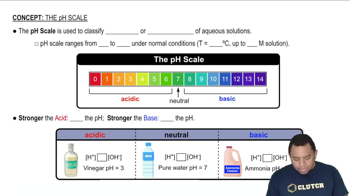

Identify the stronger base in each of the following pairs.
Explain your reasoning.
(c) HS- or OH-
Identify the stronger base in each of the following pairs.
Explain your reasoning.
(d) HS- or Br-
Calculate the H3O+ concentration to the correct number of significant figures for solutions with the following pH values. (e) -1.0
Which of the indicators given in Figure 16.5, methyl violet, bromcresol green, phenol red, or thymolphthalein, would be most appropriate to detect a pH change from: (b) 8 to 10?
Which of the indicators given in Figure 16.5, methyl violet, bromcresol green, phenol red, or thymolphthalein, would be most appropriate to detect a pH change from: (c) 2 to 0?