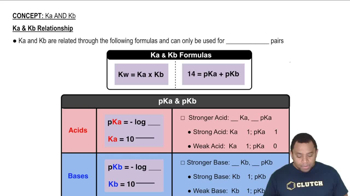
Dissociation Constants (Ka and Kb)
Dissociation constants, such as Ka for acids and Kb for bases, quantify the strength of an acid or base in solution. For HNO2, the given Ka value of 4.5 x 10^-4 indicates its tendency to donate protons. This value is essential for calculating the concentrations of HNO2 and its conjugate base NO2- in equilibrium, as well as for determining the pH of the solution.

 Verified step by step guidance
Verified step by step guidance