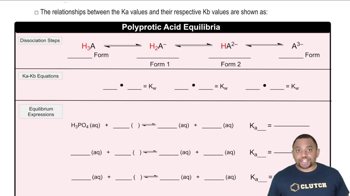
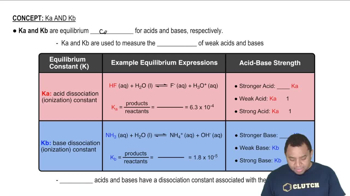
Dissociation Constants (Ka)
The dissociation constant (Ka) quantifies the strength of an acid in solution, indicating the extent to which it donates protons (H+) to water. For HF, with a given Ka of 3.5 x 10^-4, this value helps determine the equilibrium concentrations of the species present in the solution. Understanding how to apply the Ka value in conjunction with the concentrations of the acids allows for the calculation of the concentrations of H3O+, F-, and other species in the solution.

 Verified step by step guidance
Verified step by step guidance