Textbook Question
Which of the following species behave as strong acids or asstrong bases in aqueous solution?(a) HNO2 (b) HNO3(c) NH4+ (d) Cl-

 Verified step by step guidance
Verified step by step guidance


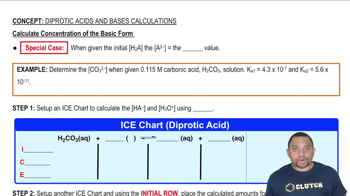
Calculate the pH of solutions prepared by: (d) Mixing 100.0 mL of 2.0 * 10-3 M HCl and 400.0 mL of 1.0 * 10-3 M HClO4. (Assume that volumes are additive.)