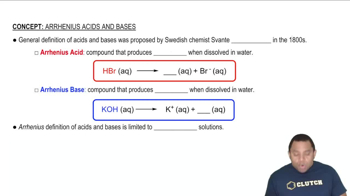
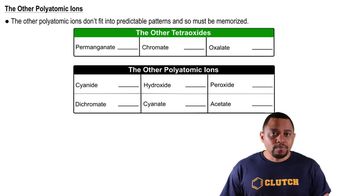
Textbook Question
Write a balanced net ionic equation for the principal reaction in solutions of each of the following salts. In each case, identify the Brønsted–Lowry acids and bases and the conjugate acid–base pairs. (a) Na2CO3

 Verified step by step guidance
Verified step by step guidance