Refining petroleum involves cracking large hydrocarbon molecules into smaller, more volatile pieces. A simple example of hydrocarbon cracking is the gas-phase thermal decomposition of butane to give ethane and ethylene: (a) Write the equilibrium constant expressions for Kp and Kc.

Consider the gas-phase decomposition of NOBr: 2 NOBr(g) ⇌ 2 NO(g) + Br2(g). (a) When 0.0200 mol of NOBr is added to an empty 1.00-L flask and the decomposition reaction is allowed to reach equilibrium at 300 K, the total pressure in the flask is 0.588 atm. What is the equilibrium constant Kc for this reaction at 300 K? (b) What is the value of Kp for this reaction at 300 K?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Equilibrium Constant (Kc)

Partial Pressure and Kp
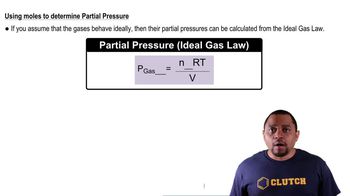
Ideal Gas Law

Refining petroleum involves cracking large hydrocarbon molecules into smaller, more volatile pieces. A simple example of hydrocarbon cracking is the gas-phase thermal decomposition of butane to give ethane and ethylene: (c) A sample of butane having a pressure of 50 atm is heated at 500 °C in a closed container at constant volume. When equilibrium is reached, what percentage of the butane has been converted to ethane and ethylene? What is the total pressure at equilibrium?
The F-F bond in F2 is relatively weak because the lone pairs of electrons on one F atom repel the lone pairs on the other F atom; Kp = 7.83 at 1500 K for the reaction F2(g) ⇌ 2 F(g). (a) If the equilibrium partial pressure of F2 molecules at 1500 K is 0.200 atm, what is the equilibrium partial pressure of F atoms in atm?
The F-F bond in F2 is relatively weak because the lone pairs of electrons on one F atom repel the lone pairs on the other F atom; Kp = 7.83 at 1500 K for the reaction F2(g) ⇌ 2 F(g). (b) What fraction of the F2 molecules dissociate at 1500 K?
