Textbook Question
Vinegar contains acetic acid, a weak acid that is partially dissociated in aqueous solution:CH3CO2H1aq2 ∆ H+ 1aq2 + CH3CO-1aq2 (b) What is the value of Kc if the extent of dissociation in1.0 M CH3CO2H is 0.42%?
1
views

 Verified step by step guidance
Verified step by step guidance
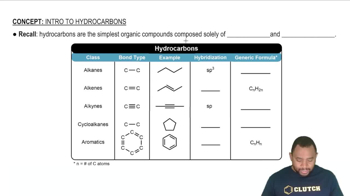

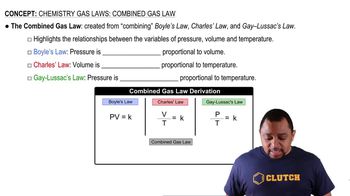
Refining petroleum involves cracking large hydrocarbon molecules into smaller, more volatile pieces. A simple example of hydrocarbon cracking is the gas-phase thermal decomposition of butane to give ethane and ethylene: (a) Write the equilibrium constant expressions for Kp and Kc.