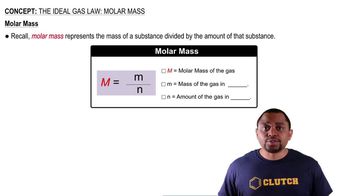
Textbook Question
Assume that you have a cylinder with a movable piston. What would happen to the gas volume of the cylinder if you were to do the following? (b) Increase the amount of gas by one-fourth while holding the temperature and pressure constant