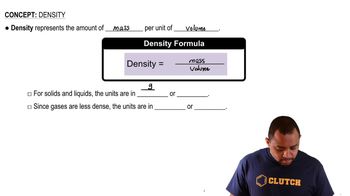
Textbook Question
Vinaigrette salad dressing consists mainly of oil and vinegar. The density of olive oil is 0.918 g/cm3, the density of vinegar is 1.006 g/cm3, and the two do not mix. If a certain mixture of olive oil and vinegar has a total mass of 397.8 g and a total volume of 422.8 cm3, what is the volume of oil and what is the volume of vinegar in the mixture?