At 273 K and 1 atm pressure, 1 mol of an ideal gas occupies 22.4 L. (Section 10.4) (c) In which parts of the atmosphere would you expect gases to behave most ideally (ignoring any photochemical reactions)? [Section 18.1]
Ch.18 - Chemistry of the Environment

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 18, Problem 5
In the following instances, which choice is greener in a chemical process and why? (b) A reagent for the reaction that can be obtained from corn husks or one that is obtained from petroleum. (c) A process that produces no by-products or one in which the by-products are recycled for another process.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of 'green chemistry', which aims to design chemical products and processes that reduce or eliminate the use and generation of hazardous substances.
Step 2: For part (b), consider the source of the reagent. A reagent obtained from corn husks is derived from a renewable resource, whereas one obtained from petroleum is derived from a non-renewable resource. Green chemistry favors the use of renewable resources.
Step 3: Evaluate the environmental impact of sourcing the reagent. Corn husks are a by-product of agriculture and using them can reduce waste, while petroleum extraction and processing have significant environmental impacts.
Step 4: For part (c), analyze the process efficiency. A process that produces no by-products is ideal as it minimizes waste. However, if by-products are generated, recycling them for another process can also be considered green as it reduces waste and resource consumption.
Step 5: Compare the overall sustainability of the options. A process with no by-products is generally more sustainable, but recycling by-products can also contribute to sustainability by creating a closed-loop system.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Green Chemistry Principles
Green chemistry focuses on designing chemical processes and products that minimize environmental impact. It emphasizes the use of renewable resources, reduction of waste, and the avoidance of hazardous substances. Understanding these principles helps evaluate the sustainability of chemical choices, such as preferring reagents derived from renewable sources like corn husks over those from non-renewable sources like petroleum.
Recommended video:
Guided course
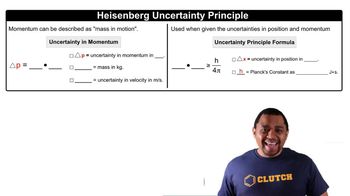
Uncertainty Principle Formula
Sustainability in Chemical Processes
Sustainability in chemical processes refers to the ability to meet current needs without compromising the ability of future generations to meet theirs. This includes using resources efficiently, minimizing waste, and ensuring that by-products can be reused or recycled. Evaluating processes based on their sustainability helps determine which options are greener, such as favoring processes that produce no by-products.
Recommended video:
Guided course

Chemical Properties Example
Life Cycle Assessment (LCA)
Life Cycle Assessment (LCA) is a systematic approach to evaluating the environmental impacts of a product or process from cradle to grave. It considers all stages, including raw material extraction, production, use, and disposal. By applying LCA, one can assess the overall environmental footprint of different chemical processes and make informed decisions about greener alternatives.
Recommended video:
Guided course
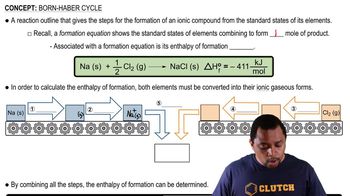
Born Haber Cycle
Related Practice
Textbook Question
Textbook Question
The figure shows the three lowest regions of Earth's atmo- sphere.
(d) An aurora borealis is due to excitation of atoms and molecules in the atmosphere 55–95 km above Earth's surface. Which regions in the figure are involved in an aurora borealis?
4
views
Textbook Question
Where does the energy come from to evaporate the esti- mated 425,000 km3 of water that annually leaves the oceans, as illustrated here? [Section 18.3]
3
views
Textbook Question
(a) What is the primary basis for the division of the atmosphere into different regions?
3
views
1
rank
Textbook Question
(a) How are the boundaries between the regions of the atmosphere determined?
1
views
