The enthalpy of evaporation of water is 40.67 kJ/mol. Sunlight striking Earth's surface supplies 168 W per square meter (1 W = 1 watt = 1 J/s). (a) Assuming that evaporation of water is due only to energy input from the Sun, calculate how many grams of water could be evaporated from a 1.00 square meter patch of ocean over a 12-h day

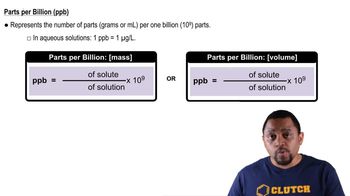
Gold is found in seawater at very low levels, about 0.05 ppb by mass. Assuming that gold is worth about \$1300 per troy ounce, how many liters of seawater would you have to process to obtain \$1,000,000 worth of gold? Assume the density of water is 1.03 g/mL and that your gold recovery process is 50% efficient.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Parts per billion (ppb)
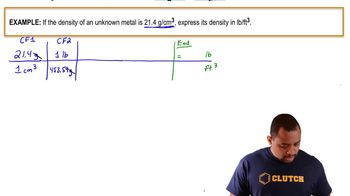
Density and volume conversion
Efficiency of recovery processes
The enthalpy of fusion of water is 6.01 kJ/mol. Sunlight striking Earth's surface supplies 168 W per square meter (1 W = 1 watt = 1 J/s). (b) The specific heat capacity of ice is 2.032 J/g°C. If the initial temperature of a 1.00 square emter patch of ice is -5.0°C, what is its final temperature after being in sunlight for 12 h, assuming no phase changes and assuming that sunlight penetration uniformly to a depth of 1.00 cm?
The Ogallala aquifer described in the Closer Look box in Section 18.3, provides 82% of the drinking water for the people who live in the region, although more than 75% of the water that is pumped from it is for irrigation. Irrigation withdrawals are approximately 18 billion gallons per day. (a) Assuming that 2% of the rainfall that falls on an area of 600,000 km2 recharges the aquifer, what average annual rainfall would be required to replace the water removed for irrigation?
The organic anion
is found in most detergents. Assume that the anion under-goes aerobic decomposition in the following manner: C18H29SO3- + 51 O2 → 36 CO2(aq) + 28 H2O (l) + 2 H+(aq) + 2 SO42-(aq) What is the total mass of O2 required to biodegrade 10.0 g of this substance?
