What is the molarity of Na+ in a solution of NaCl whose salinity is 5.6 if the solution has a density of 1.03 g>mL?
Ch.18 - Chemistry of the Environment

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 18, Problem 37a
The enthalpy of evaporation of water is 40.67 kJ/mol. Sunlight striking Earth's surface supplies 168 W per square meter (1 W = 1 watt = 1 J/s). (a) Assuming that evaporation of water is due only to energy input from the Sun, calculate how many grams of water could be evaporated from a 1.00 square meter patch of ocean over a 12-h day
 Verified step by step guidance
Verified step by step guidance1
Convert the power supplied by sunlight from watts to joules over the 12-hour period. Use the formula: \( \text{Energy (J)} = \text{Power (W)} \times \text{Time (s)} \).
Calculate the total energy supplied by the Sun to the 1.00 square meter patch of ocean over 12 hours. Remember that 12 hours is equivalent to 43,200 seconds.
Determine the number of moles of water that can be evaporated using the total energy calculated and the enthalpy of evaporation. Use the formula: \( \text{Moles of water} = \frac{\text{Total energy (J)}}{\text{Enthalpy of evaporation (J/mol)}} \).
Convert the moles of water to grams using the molar mass of water (18.02 g/mol). Use the formula: \( \text{Mass (g)} = \text{Moles} \times \text{Molar mass (g/mol)} \).
Summarize the steps to find the total mass of water that can be evaporated from the given area over the specified time period.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Enthalpy of Evaporation
The enthalpy of evaporation, or heat of vaporization, is the amount of energy required to convert a unit mass of a liquid into vapor without a change in temperature. For water, this value is 40.67 kJ/mol, indicating that this amount of energy is needed to evaporate one mole of water. Understanding this concept is crucial for calculating how much water can be evaporated given a specific energy input.
Recommended video:
Guided course
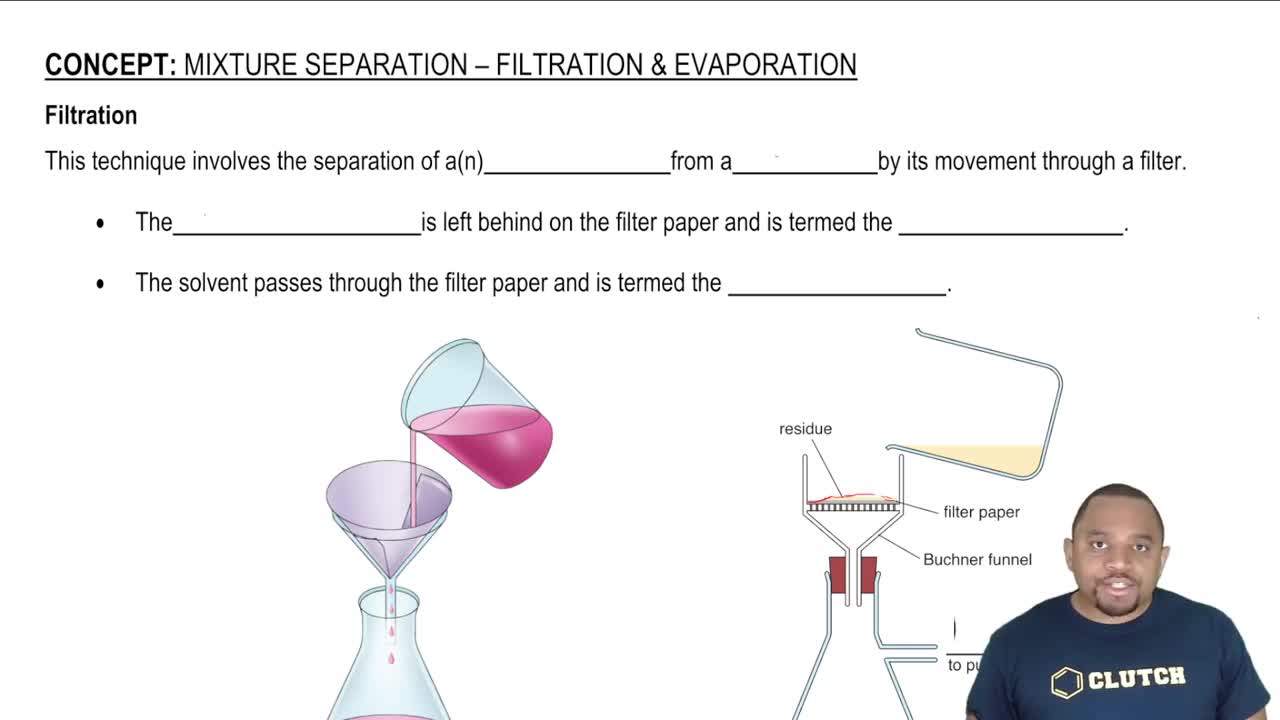
Filtration and Evaporation
Power and Energy Conversion
Power is the rate at which energy is transferred or converted, measured in watts (W), where 1 W equals 1 joule per second (J/s). In this context, sunlight provides energy at a rate of 168 W per square meter. To find the total energy available over a period, one must multiply the power by the time duration, converting hours into seconds for accurate calculations.
Recommended video:
Guided course

Conversion Factors
Mass-Energy Relationship
The mass-energy relationship in chemistry allows us to relate the energy absorbed during a phase change to the mass of the substance undergoing that change. By using the enthalpy of evaporation, we can determine how many grams of water can be evaporated by dividing the total energy available (calculated from power and time) by the energy required to evaporate a specific mass of water. This relationship is fundamental for solving the problem presented.
Recommended video:
Guided course
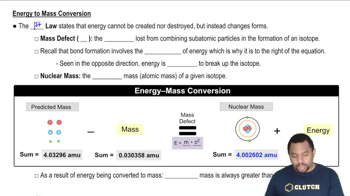
Energy to Mass Conversion
Related Practice
Textbook Question
25
views
Textbook Question
Phosphorus is present in seawater to the extent of 0.07 ppm by mass. Assuming that the phosphorus is present as dihydrogenphosphate, H2PO4-, calculate the correspond-ing molar concentration of H2PO4- in seawater.
Textbook Question
The enthalpy of fusion of water is 6.01 kJ/mol. Sunlight striking Earth's surface supplies 168 W per square meter (1 W = 1 watt = 1 J/s). (b) The specific heat capacity of ice is 2.032 J/g°C. If the initial temperature of a 1.00 square emter patch of ice is -5.0°C, what is its final temperature after being in sunlight for 12 h, assuming no phase changes and assuming that sunlight penetration uniformly to a depth of 1.00 cm?
2
views
