(a) Based on the lattice energies of MgCl2 and SrCl2 given in Table 8.1, what is the range of values that you would expect for the lattice energy of CaCl2?

Which of these elements are unlikely to form ionic bonds: Mg, Al, Si, Br, I?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Ionic Bonds
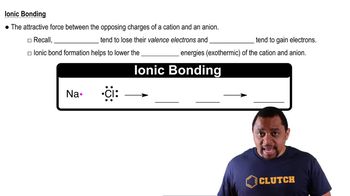
Electronegativity

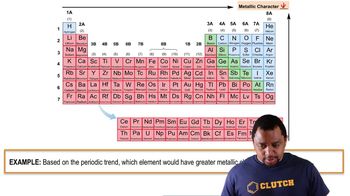
Metallic vs. Nonmetallic Character
(b) Using data from Appendix C, Figure 7.11, Figure 7.13, and the value of the second ionization energy for Ca, 1145 kJ/mol, calculate the lattice energy of CaCl2.
(b) A substance, XY, formed from two different elements, melts at −33 °C. Is XY likely to be a covalent or an ionic substance?
Using Lewis symbols and Lewis structures, diagram the formation of SiCl4 from Si and Cl atoms, showing valence-shell electrons. e. How many bonding pairs of electrons are in the SiCl4 molecule?
Using Lewis symbols and Lewis structures, diagram the formation of PF3 from P and F atoms, showing valence-shell electrons. (a) How many valence electrons does P have initially? (c) How many valence electrons surround the P in the PF3 molecule? (d) How many valence electrons surround each P in the PF3 molecule?
Use Lewis symbols and Lewis structures to diagram the formation of PF3 from P and F atoms, showing valence-shell electrons. e. How many bonding pairs of electrons are in the PF3 molecule?
