(b) Using data from Appendix C, Figure 7.11, Figure 7.13, and the value of the second ionization energy for Ca, 1145 kJ/mol, calculate the lattice energy of CaCl2.
Ch.8 - Basic Concepts of Chemical Bonding

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 8, Problem 35e
Using Lewis symbols and Lewis structures, diagram the formation of SiCl4 from Si and Cl atoms, showing valence-shell electrons. e. How many bonding pairs of electrons are in the SiCl4 molecule?
 Verified step by step guidance
Verified step by step guidance1
Start by identifying the valence electrons for each atom. Silicon (Si) is in group 14 and has 4 valence electrons. Chlorine (Cl) is in group 17 and has 7 valence electrons.
Draw the Lewis symbol for Si with its 4 valence electrons represented as dots around the symbol Si.
Draw the Lewis symbol for Cl with its 7 valence electrons represented as dots around the symbol Cl. Repeat this for four Cl atoms.
To form SiCl4, each Cl atom will share one electron with Si, forming a single covalent bond. Draw a line between Si and each Cl to represent these bonds.
Count the number of bonding pairs of electrons. Each line represents a pair of shared electrons, so there are four bonding pairs in SiCl4.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Symbols
Lewis symbols represent the valence electrons of an atom as dots surrounding the element's symbol. Each dot corresponds to a valence electron, which is crucial for understanding how atoms bond. In the case of silicon (Si) and chlorine (Cl), the Lewis symbols help visualize the number of valence electrons available for bonding, which is essential for constructing Lewis structures.
Recommended video:
Guided course
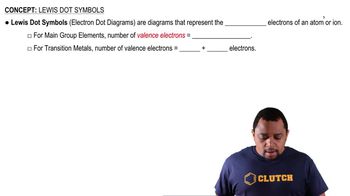
Lewis Dot Symbols
Lewis Structures
Lewis structures are diagrams that depict the arrangement of atoms and the distribution of valence electrons in a molecule. They illustrate bonding pairs (shared electrons) and lone pairs (non-bonding electrons) around atoms. For SiCl4, the Lewis structure shows how silicon shares its four valence electrons with four chlorine atoms, forming single covalent bonds.
Recommended video:
Guided course

Lewis Dot Structures: Ions
Bonding Pairs of Electrons
Bonding pairs of electrons are pairs of electrons that are shared between two atoms, forming a covalent bond. In SiCl4, each Si-Cl bond consists of one bonding pair, resulting in a total of four bonding pairs for the molecule. Understanding the concept of bonding pairs is essential for determining the molecular structure and properties of compounds.
Recommended video:
Guided course
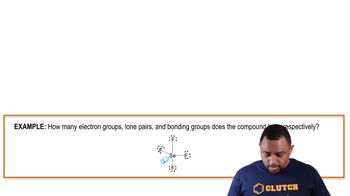
Electron Groups, Lone Pairs, and Bonding Groups Example
Related Practice
Textbook Question
Textbook Question
(b) A substance, XY, formed from two different elements, melts at −33 °C. Is XY likely to be a covalent or an ionic substance?
1
views
Textbook Question
Using Lewis symbols and Lewis structures, diagram the formation of PF3 from P and F atoms, showing valence-shell electrons. (a) How many valence electrons does P have initially? (c) How many valence electrons surround the P in the PF3 molecule? (d) How many valence electrons surround each P in the PF3 molecule?
Textbook Question
Use Lewis symbols and Lewis structures to diagram the formation of PF3 from P and F atoms, showing valence-shell electrons. e. How many bonding pairs of electrons are in the PF3 molecule?
Textbook Question
(b) How many bonding electrons are in the structure?
