Two compounds are isomers if they have the same chemical formula but different arrangements of atoms. Use Table 8.3 to estimate H for each of the following gas-phase isomerization reactions and indicate which isomer has the lower enthalpy. (d) Methyl isocyanide → Acetonitrile

The compound chloral hydrate, known in detective stories as knockout drops, is composed of 14.52% C, 1.83% H, 64.30% Cl, and 13.35% O by mass, and has a molar mass of 165.4 g/mol. (c) Draw the Lewis structure of the molecule, assuming that the Cl atoms bond to a single C atom and that there are a C–C bond and two C–O bonds in the compound.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Molecular Composition and Percent Composition
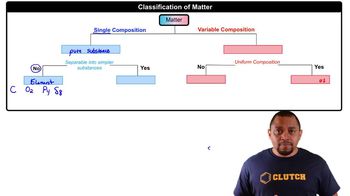
Molar Mass and Molecular Formula

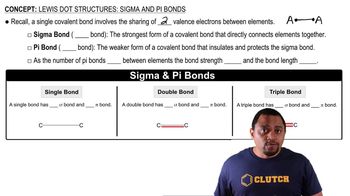
Lewis Structures and Bonding
The electron affinity of oxygen is -141 kJ/mol, corresponding to the reaction O(g) + e- → O-(g). The lattice energy of K2O(s) is 2238 kJ/mol. Use these data along with data in Appendix C and Figure 7.11 to calculate the 'second electron affinity' of oxygen, corresponding to the reaction O-(g) + e- → O2-(g)
Under special conditions, sulfur reacts with anhydrous liquid ammonia to form a binary compound of sulfur and nitrogen. The compound is found to consist of 69.6% S and 30.4% N. Measurements of its molecular mass yield a value of 184.3 g/mol. The compound occasionally detonates on being struck or when heated rapidly. The sulfur and nitrogen atoms of the molecule are joined in a ring. All the bonds in the ring are of the same length. (a) Calculate the empirical and molecular formulas for the substance.
A common form of elemental phosphorus is the tetrahedral P4 molecule, where all four phosphorus atoms are equivalent:
(b) How many P-P bonds are there in the molecule?
A common form of elemental phosphorus is the tetrahedral P4 molecule, where all four phosphorus atoms are equivalent:
Draw a Lewis structure for a linear P4 molecule that satisfies the octet rule. Does this molecule have resonance structures?
