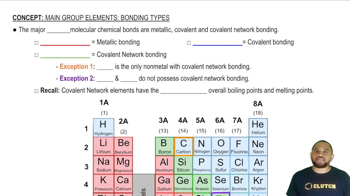
The following three Lewis structures can be drawn for N2O:
(a) Using formal charges, which of these three resonance forms is likely to be the most important?

 Verified step by step guidance
Verified step by step guidance

The following three Lewis structures can be drawn for N2O:
(a) Using formal charges, which of these three resonance forms is likely to be the most important?
The following three Lewis structures can be drawn for N2O:
(b) The N—N bond length in N2O is 1.12 Å, slightly longer than a typical N≡N bond; and the N—O bond length is 1.19 Å, slightly shorter than a typical N═O bond (see Table 8.4). Based on these data, which resonance structure best represents N2O?
Mothballs are composed of naphthalene, C10H8, a molecule that consists of two six-membered rings of carbon fused along an edge, as shown in this incomplete Lewis structure:(a) Draw all of the resonance structures of naphthalene. How many are there?
Mothballs are composed of naphthalene, C10H8, a molecule that consists of two six-membered rings of carbon fused along an edge, as shown in this incomplete Lewis structure:
(c) Not all of the C—C bond lengths in naphthalene are equivalent. Based on your resonance structures, how many C—C bonds in the molecule do you expect to be shorter than the others?
a. Triazine, C3H3N3, is like benzene except that in triazine every other C—H group is replaced by a nitrogen atom. Draw the Lewis structure(s) for the triazine molecule.