The hypochlorite ion, ClO-, is the active ingredient in bleach. The perchlorate ion, ClO4-, is a main component of rocket propellants. Draw Lewis structures for both ions. (b) What is the formal charge of Cl in the perchlorate ion, assuming the Cl—O bonds are all single bonds?

Mothballs are composed of naphthalene, C10H8, a molecule that consists of two six-membered rings of carbon fused along an edge, as shown in this incomplete Lewis structure:(a) Draw all of the resonance structures of naphthalene. How many are there?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Resonance Structures
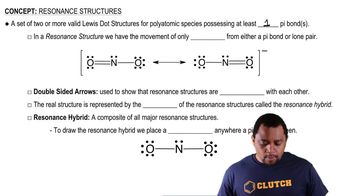
Lewis Structures

Delocalization of Electrons
The following three Lewis structures can be drawn for N2O:
(a) Using formal charges, which of these three resonance forms is likely to be the most important?
The following three Lewis structures can be drawn for N2O:
(b) The N—N bond length in N2O is 1.12 Å, slightly longer than a typical N≡N bond; and the N—O bond length is 1.19 Å, slightly shorter than a typical N═O bond (see Table 8.4). Based on these data, which resonance structure best represents N2O?
Mothballs are composed of naphthalene, C10H8, a molecule that consists of two six-membered rings of carbon fused along an edge, as shown in this incomplete Lewis structure:
(b) Do you expect the C—C bond lengths in the molecule to be similar to those of C—C single bonds, C ═C double bonds, or intermediate between C—C single and C ═C double bonds?
Mothballs are composed of naphthalene, C10H8, a molecule that consists of two six-membered rings of carbon fused along an edge, as shown in this incomplete Lewis structure:
(c) Not all of the C—C bond lengths in naphthalene are equivalent. Based on your resonance structures, how many C—C bonds in the molecule do you expect to be shorter than the others?
