An element X reacts with oxygen to form XO2 and with chlorine to form XCl4. XO2 is a white solid that melts at high temperatures (above 1000 °C). Under usual conditions, XCl4 is a colorless liquid with a boiling point of 58 °C. (b) Do you think that element X is a metal, nonmetal, or metalloid?
Ch.7 - Periodic Properties of the Elements

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 7, Problem 66d
Write balanced equations for the following reactions. d. selenium dioxide with aqueous potassium hydroxide.
 Verified step by step guidance
Verified step by step guidance1
Identify the reactants: Selenium dioxide (SeO₂) and aqueous potassium hydroxide (KOH).
Determine the products: When selenium dioxide reacts with potassium hydroxide, it typically forms potassium selenite (K₂SeO₃) and water (H₂O).
Write the unbalanced chemical equation: SeO₂ + KOH → K₂SeO₃ + H₂O.
Balance the equation by adjusting coefficients to ensure the same number of each type of atom on both sides of the equation.
Verify that the equation is balanced by counting the atoms of each element on both sides and ensuring they are equal.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations involves ensuring that the number of atoms for each element is the same on both the reactant and product sides. This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. To balance an equation, coefficients are adjusted in front of the chemical formulas without changing the actual formulas themselves.
Recommended video:
Guided course

Balancing Chemical Equations
Acid-Base Reactions
Acid-base reactions typically involve the transfer of protons (H+) between reactants. In this case, potassium hydroxide (KOH) acts as a strong base, while selenium dioxide (SeO2) can behave as an acid in the presence of water. Understanding the nature of these reactants helps predict the products formed in the reaction, which often include water and a salt.
Recommended video:
Guided course
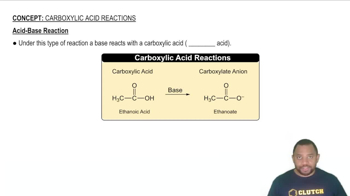
Acid-Base Reaction
Selenium Compounds
Selenium dioxide (SeO2) is an important selenium compound that can react with bases to form selenites. In aqueous solutions, it can interact with hydroxide ions from potassium hydroxide, leading to the formation of selenite ions. Recognizing the properties and reactivity of selenium compounds is crucial for predicting the outcome of the reaction and writing the balanced equation.
Recommended video:
Guided course

Hydrogen Compounds Example
Related Practice
Textbook Question
Textbook Question
Write balanced equations for the following reactions. a. barium oxide with water
Textbook Question
Write balanced equations for the following reactions. c. sulfur trioxide with water
Textbook Question
Write a balanced equation for the reaction that occurs in each of the following cases: (a) Potassium metal is exposed to an atmosphere of chlorine gas.
1
views
Textbook Question
Write a balanced equation for the reaction that occurs in each of the following cases: (b) Strontium oxide is added to water. (c) A fresh surface of lithium metal is exposed to oxygen gas. (d) Sodium metal reacts with molten sulfur.
1
views
Textbook Question
Write a balanced equation for the reaction that occurs in each of the following cases: (a) Cesium is added to water.
1
views
